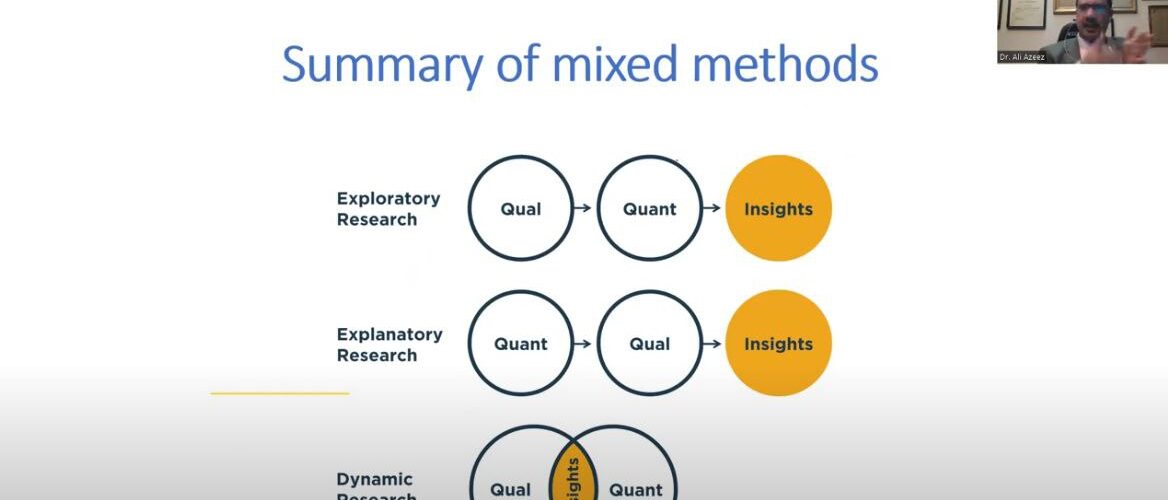
The Faculty of Pharmacy discussed the Master’s Degree thesis, “Assessment of Adverse Drug Reactions Related to Cardiac Disorders in Iraqi Public Sector: A Mixed Method Study “by Basma Osama Salman and the supervisor, Dr. Mohamed Y. Jamal, Dr. Manal M. Younus, in the Clinical Pharmacy Department. Objectives: The primary aim of the study was to determine the drugs that cause cardiac adverse drug reaction and to assess the demographic distribution, adverse drug reactions classification, severity, expectedness, preventability, and seriousness. The secondary aim of this study was explore the experience of physicians about the medicines that have adverse reactions on heart and what are the appropriate methods to prevent them. Methods: The study had a mixed-methods design with a quantitative phase and a qualitative phase. The quantitative phase included a retrospective assessment of the individual case safety reports obtained from the database of the Iraqi Pharmacovigilance Center (sent for the time period ranging from January 1, 2010, to December 31, 2021). The qualitative research involved interviews that were face-to-face, semi-structured, and had open-ended questions. The interviews were conducted in the time period from 1May 2022 to 30 June 2022, in public hospitals and private clinics. Face-to-face interviews lasted for 15 to 30 minutes, and data collection ended after the data reached saturation, the point when “no new information or themes are observed in the data.” Results: The quantitative phase included 1101 individual case safety reports that were extracted from the database containing 2453 adverse drug reactions.Then, 1303 noncardiac adverse drug reactions were excluded, and 1150 adverse drug reactions were included as cardiac reactions. Most cardiac adverse drug reactions were considered mild in severity, expected, not serious and preventable reactions. The qualitative phase included 20 specialists (13 males and 7 females) who made up the research sample from three governmental hospitals in Baghdad, Iraq. The main themes that emerged from the interviews were monitoring of cardiac adverse drug reactions, reversibility and seriousness of cardiac reactions, prevention of cardiac reactions, minimization of cardiac adverse drug reactions, treatment of cardiac adverse reactions and reporting of cardiac reactions to the Iraqi Pharmacovigilance Center. Conclusions: Adults were the most common age group to experience cardiac ADRs. Most cardiac ADRs were mild, expected, not serious, and probably preventable. Monitoring of cardiac ADRs was performed using ECG, CTG, and echo. Pharmacists’ reporting to pharmacovigilance was more than that of physicians’. Early detection of drug-induced cardiac adverse reactions, will aid in reducing long-term morbidity and mortality rates, preventing permanent heart damage, and improving patient quality of life.